- HOME
- About us
-
Product
ProductDetailSimple substanceTellurideSputtering targetiodidefluoridephosphidesulfidechlorideCutting-edge materialsselenidebromideoxide
-
 Molybdenum telluride
Molybdenum telluride
-
 Tin telluride
Tin telluride
-
 Silver telluride
Silver telluride
-
 Tellurium antimony germanium
Tellurium antimony germanium
-
 cuprous telluride
cuprous telluride
-
 Bismuth telluride
Bismuth telluride
-
 Cadmium telluride
Cadmium telluride
-
 Gallium Telluride
Gallium Telluride
-
 Aluminum iodide(AlI3)
Aluminum iodide(AlI3)
-
 STRONTIUM IODIDE
STRONTIUM IODIDE
-
 Hafnium(IV) iodide
Hafnium(IV) iodide
-
 Tetra Tin(IV) iodide
Tetra Tin(IV) iodide
-
 Chromium triiodide
Chromium triiodide
-
 Lead iodide
Lead iodide
-
 Cesium Iodide
Cesium Iodide
-
 Cuprous iodide
Cuprous iodide
-
 Barium Fluoride
Barium Fluoride
-
 Aluminum fluoride
Aluminum fluoride
-
 Magnesium fluoride
Magnesium fluoride
-
 Cerium fluoride
Cerium fluoride
-
 Ytterbium fluoride
Ytterbium fluoride
-
 Indium trifluoride
Indium trifluoride
-
 Hafnium disulfide
Hafnium disulfide
-
 Antimony Trisulfide
Antimony Trisulfide
-
 Tin monosulfide
Tin monosulfide
-
 Germanium monosulfide
Germanium monosulfide
-
 Zirconium sulfide
Zirconium sulfide
-
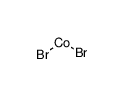
 Cobalt disulfide
Cobalt disulfide
-
 Silicon disulfide
Silicon disulfide
-
 molybdenum disulfide
molybdenum disulfide
-
 Chromium(II) Chloride
Chromium(II) Chloride
-
 Bismuth Chloride
Bismuth Chloride
-
 Palladium Chloride
Palladium Chloride
-
 Ruthenium chloride
Ruthenium chloride
-
 Ferric trichloride
Ferric trichloride
-
 Tellurium tetrachloride
Tellurium tetrachloride
-
 Hafnium tetrachloride
Hafnium tetrachloride
-
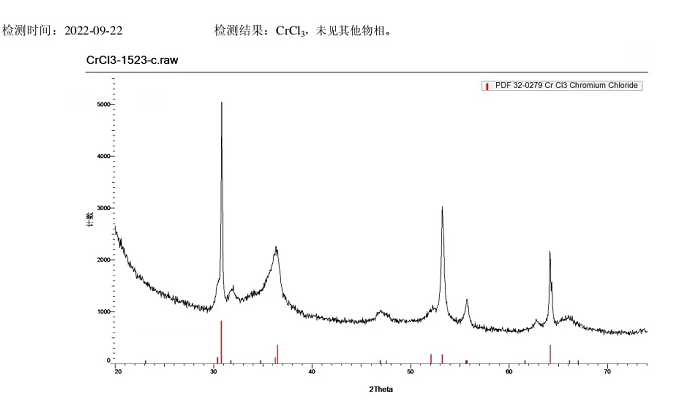 Chromium trichloride
Chromium trichloride
-
 Cadmium arsenide
Cadmium arsenide
-
 Hydrated Indium(III) nitrate
Hydrated Indium(III) nitrate
-
 Gallium nitride powder, target material
Gallium nitride powder, target material
-
 Indium acetate
Indium acetate
-
 Tellurium cadmium zinc
Tellurium cadmium zinc
-
 CIGS
CIGS
-
 Copper zinc tin sulfur
Copper zinc tin sulfur
-
 Cadmium stannate
Cadmium stannate
-
 Molybdenum diselenide
Molybdenum diselenide
-
 Titanium selenide
Titanium selenide
-
 tungsten diselenide
tungsten diselenide
-
 Gallium trislenide
Gallium trislenide
-
 Antimony triselenide
Antimony triselenide
-
 indium selenide
indium selenide
-
 bismuth selenide
bismuth selenide
-
 CdSe
CdSe
-
- Process Route
-
Technological Innovation
Technological InnovationDetail
-
Partners
PartnersDetail
-
Cooperation Case
Cooperation CaseDetail
- CONTACT US